Abstract
BACKGROUND:
In advanced systemic mastocytosis (SM), the response of neoplastic mast cells (MCs) to conventional drugs is poor and prognosis is bad. Despite the recent approval of the tyrosine kinase inhibitor midostaurin, there is still a need for targeted agents for patients (pts) who relapse or have resistant disease. Given the rarity of advanced SM, discovering novel indications for approved drugs or for drugs in phase I-II clinical development would ensure the fastest transition from bench to bed.
Polo-like kinase 1 (Plk1) and Aurora Kinase A (AKA) are master regulators of cell cycle progression and mitosis. They are frequently overexpressed in human cancers and are both being explored as therapeutic targets in clinical trials in solid tumors, myeloid leukemias and myelodysplasias.
AIMS:
In this study, we aimed to analyze the expression and functional status of Plk1 and AKA in SM and to evaluate their role as potential therapeutic targets.
METHODS:
Experiments were conducted in the HMC-1 MC leukemia cell line (two subclones: HMC-1.1, which harbors the KIT V560G but not the KIT D816V mutation, and HMC-1.2, which harbors both activating mutations) and in primary neoplastic MCs obtained from 7 SM patients (indolent SM, n=3; aggressive SM, n=3 and MC leukemia, n=1). Protein expression and activation was assessed by Western Blotting (WB). Danusertib and Volasertib were used to investigate the effects of AKA and Plk1 inhibition. Briefly, HMC-1.1 and -1.2 cells were incubated with increasing concentrations of Danusertib and Volasertib (50-100 nM) or control medium for 24, 48, 72 and 96 hours (h) to perform growth curve experiments. The percentage of apoptotic cells was quantified by trypan blue staining. To confirm apoptosis, cells were analyzed by annexin V/propidium iodide staining and flow cytometry. For evaluation of caspase cleavage, HMC-1 cells were incubated with Danusertib and Volasertib (100 nM) or control medium for 24h. WB was performed using polyclonal antibodies against cleaved caspase-3, -8 and -9. The effects of Danusertib and Volasertib on cell cycle progression were assessed by flow cytometry. Wee 1 inhibitor MK1775 was also tested alone or in combination with Danusertib and Volasertib at nanomolar doses (500 nM). Clonogenic assays were performed to confirm cytostatic effects of the drugs administrated alone or in combination.
RESULTS:
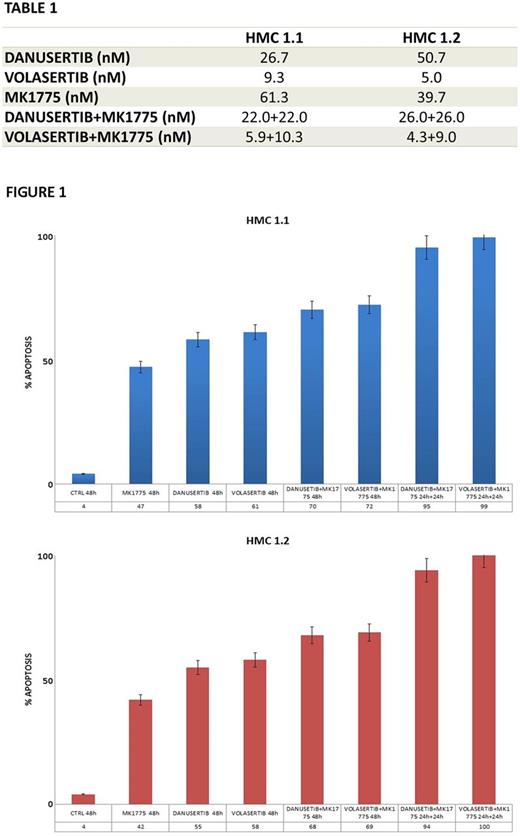
Primary neoplastic MCs as well as HMC-1 cells displayed hyper-phosphorylated PLK-1 and AKA. Danusertib and Volasertib inhibited growth and induced apoptotic cell death in HMC-1.1 (IC50=649nM and 443nM, respectively) and -1.2 cells (IC50=892nM and 808nM, respectively). The growth-inhibitory effects of Danusertib and Volasertib were found to be associated with mitotic arrest and activation of apoptosis. Cell cycle arrest was associated with increased levels of phospho (p)-Chk1 and p-Chk2, p-cyclin B1, p-cdc2 and p-Wee1. Apoptosis was demonstrated by an increase of annexin-V-positive cells and by the detection of the cleaved forms of caspase-3, -8 and -9. Sub-lethal drug doses were established by using a dose escalation for 24h and after interpolation of results with dose-response curves the IC50 values calculated using Compusyn were: HMC-1.1 - 880nM for MK1775, 310+310nM for danusertib+MK1775, 300+300nM for volasertib+MK1775; HMC-1.2 - 1254nM for MK1775, 397+397nM for danusertib+MK1775, 322+322nM for volasertib+MK1775. Incubation with MK1775 (500 nM) after 24h treatment with Danusertib or Volasertib (100nM), when cells were arrested in G2 phase and Wee1 was overexpressed and hyper-activated, resulted in a percentage of apoptotic cells significantly higher than that obtained from concomitant treatment with Danusertib or Volasertib (100nM) + MK1775 (500 nM) for 48h. Finally, Danusertib and Volasertib were found to synergize with MK1775 in inhibiting HMC-1 clonogenic potential (Table 1) and in inducing apoptotic cell death (Figure 1). Volasertib or Danusertib±MK1775 did not induce apoptosis in normal cultured cells.
CONCLUSIONS:
PLK1-1 and AKA, alone or together with Wee1, are attractive therapeutic targets in neoplastic MCs. Repurposing PLK1 or AKA±Wee1 inhibitors in advanced clinical development for other indications is a therapeutic strategy worth to be explored in an attempt to improve the outcome of patients with advanced SM.
Supported by AIL and AIRC (16996)
Zanotti: Deciphera: Consultancy. Valent: Celgene: Honoraria, Research Funding; Blueprint: Research Funding; Novartis: Honoraria, Research Funding; Incyte: Honoraria; Teva: Honoraria; Pfizer: Honoraria; Deciphera: Honoraria, Research Funding; Ariad: Honoraria, Research Funding; BMS: Honoraria. Martinelli: Ariad/Incyte: Consultancy; Pfizer: Consultancy; Celgene: Consultancy; Amgen: Consultancy; Johnson&Johnson: Consultancy; Roche: Consultancy. Soverini: Incyte Biosciences: Consultancy; Bristol-Myers Squibb: Consultancy; Novartis: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal